Advanced Targeting.
Designed to Combine.
Precigen advances the promise of precision immunology through novel gene and cell therapies designed to work in combination to achieve efficacy and safety.

AdenoVerse
The AdenoVerse® Advantage
- Large payload capacity
- Low seroprevalance in humans
- Ability for repeat administration
- Durable antigen-specific immune response
- Highly productive manufacturing process
“Off-the-shelf” AdenoVerse Gene Therapy / Cytokine Therapy
The AdenoVerse platform utilizes a library of proprietary adenovectors for the efficient gene delivery of vaccine antigens and cytokines to modulate the immune system. Our proprietary gorilla adenovectors are potent, have high payload capacity and are amenable to repeated patient administration. AdenoVerse therapies are manufactured using proprietary manufacturing cell lines and easily scalable production methodology. Superior performance characteristics and high yield manufacturing of AdenoVerse vectors combined with UltraVector technology allow us to engineer cutting-edge gene therapies to treat complex diseases.
Large genetic payload capacity
Our gorilla adenovectors can deliver up to 12kb of genetic payload in vivo, which is larger than other viral vectors currently ubiquitous in the gene therapy field. This allows us to engineer gene therapies that express multiple genes in a controlled manner.
Repeat administration
Our gorilla adenovectors have very low seroprevalance in humans and thus are more suitable for repeated administration. Ability to redose patients is a major advantage of AdenoVerse since it can lead to boosted antibody and T cell responses and improved outcomes for patients.
Non-replicating adenoviruses
AdenoVerse therapies are engineered to be replication deficient in vivo, and do not exhibit cytopathic or cytotoxic effects in normal human cells. Lack of in vivo replication and high yield manufacturing process reduces the regulatory and commercialization risks.
Durable antigen-specific immune response
Gorilla adenovectors engineered to present particular antigens have been shown to generate a high-level of durable antigen-specific neutralizing antibodies and effector T cell immune responses as well as an ability to boost these antibody and T cell responses via repeat administration in animal models.
We are developing multiple AdenoVerse therapies for immuno-oncology and infectious disease areas.

UltraCAR-T
The UltraCAR-T® Advantage
- Non-viral multi-gene delivery
- Overnight manufacturing process
- Higher antigen-specific expansion and in vivo persistence
- Non-exhausted, stem-like T cell phenotype
- Ability to deplete with integrated kill switch
CAR-T cell therapies require new approaches for broader patient access
Chimeric antigen receptor T, or CAR-T, cell therapies have shown remarkable responses in patients with Hematologic B cell cancers; however, high cost and long delays due to complex and lengthy manufacturing processes are major obstacles to their broad adoption. Furthermore, autologous and allogeneic CAR-T cell therapies face challenges in the treatment of solid tumors due to their rapid exhaustion and limited persistence, which limits the duration of their anti-tumor response in the body.
UltraCAR-T: a new class of CAR-T cell therapy
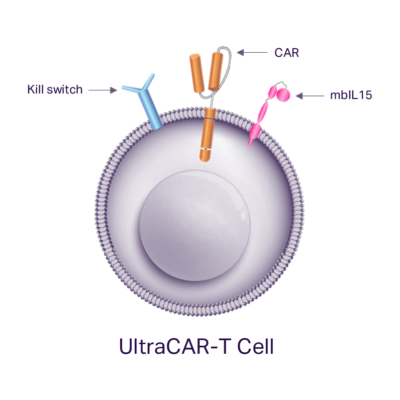
The UltraCAR-T platform is fundamentally differentiated from the competition and has the potential to disrupt the CAR-T treatment landscape by increasing patient access through rapid manufacturing, lower manufacturing-related costs, and improved outcomes using advanced technologies for precise tumor targeting and control of the immune system.
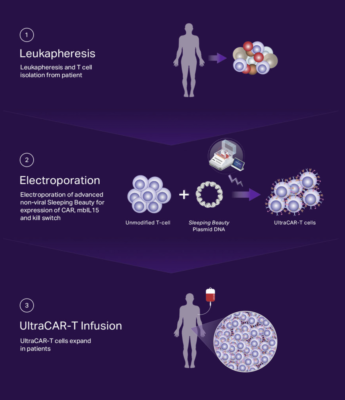
Non-viral multigenic delivery system
UltraCAR-T cells utilize the non-viral Sleeping Beauty system, which has been optimized using our UltraVector® DNA construction platform to deliver a large multigenic payload at high efficiency. As a result, UltraCAR-T cells are precision-engineered to produce a homogeneous cell product that simultaneously expresses antigen-specific CAR, kill switch, and mbIL15 genes. Antigen binding, hinge and signaling domains of each CAR are optimized for tumor cell killing based on the target antigen expression profile. Every UltraCAR-T cell is equipped with our proprietary kill switch technology to enable their controlled elimination to improve the safety profile.
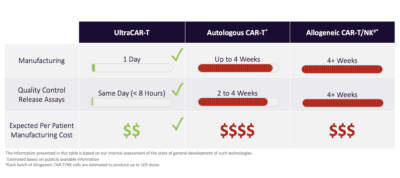
Precigen’s UltraCAR-T is the only platform to significantly reduce manufacturing and treating time for autologous CAR-T to just one day.
In vivo expansion and persistence
The key driver of improved UltraCAR-T performance is the expression of our proprietary membrane-bound interleukin-15, or mbIL15. IL-15 is a master regulator cytokine that promotes T-cell activation and expansion as well as survival of memory T cells to enhance anti-tumor response. mbIL15 is tethered to the cell surface and functions locally to enhance the functionality of the UltraCAR-T cell without systemic delivery of IL-15. Expression of mbIL15 is shown to enhance in vivo expansion in the presence of tumor antigens and prevent UltraCAR-T cell exhaustion leading to longer persistence and an enduring anti-tumor response that outlasts conventional CAR-T cells.
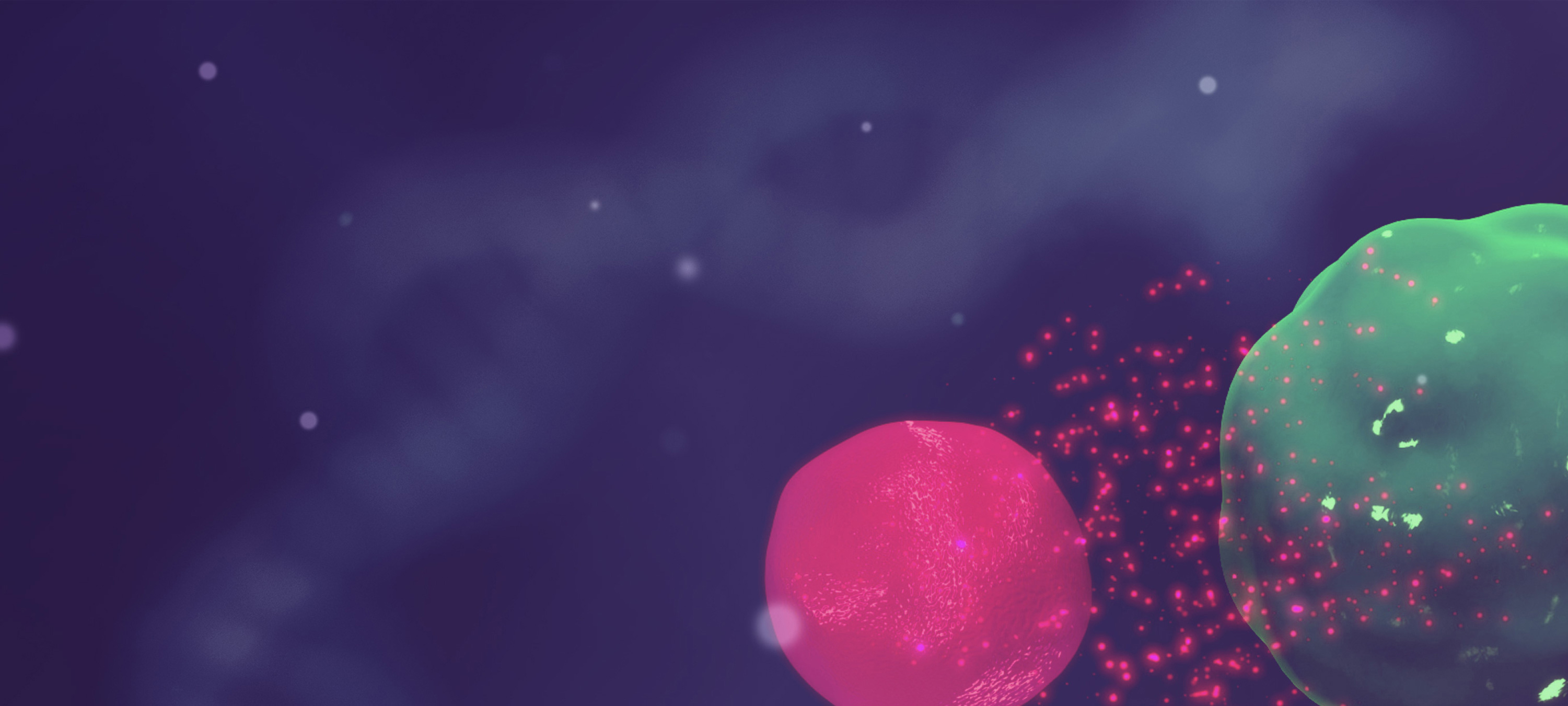
Conventional CAR-T cells struggle to contain growth of aggressive solid tumor cells (as expressed in green fluorescent protein) in a long-term in vitro culture consistent with their limited potential for persistence due to exhaustion. In stark contrast, UltraCAR-T cells exhibit sustained killing of tumor cells and inhibit tumor growth highlighting their potential for an enduring anti-tumor response.
Rapid, decentralized manufacturing process
UltraCAR-T cells are manufactured by a rapid, streamlined manufacturing process that forgoes the need for large, centralized facilities, lengthy manufacturing process resulting in exhaustion and limited in vivo life-span of current CAR-T cells and contributes to the high costs of therapies. Instead, UltraCAR-T manufacturing requires isolation of the patient’s own T cells after blood draw, followed by non-viral gene transfer using plasmid DNA at medical centers. The next day following the gene transfer, UltraCAR-T cells are infused into the patient. The UltraCAR-T manufacturing process can scale beyond the confines of a dedicated facility and provides a significant potential competitive advantage in the timeline and cost required to manufacture and deliver CAR-T therapies to patients.
We are currently evaluating three UltraCAR-T treatments in clinical trials. PRGN-3005 UltraCAR-T is an investigational therapy for advanced ovarian cancer; PRGN-3006 is an investigational therapy for relapsed or refractory AML and higher-risk MDS; and PRGN-3007, based on the next generation UltraCAR-T, is an investigational therapy targeting ROR+ hematological and solid tumors. We are now developing a library of UltraCAR-T to target various tumor antigens with the goal of providing personalized, autologous CAR-T treatment for cancer patients in a rapid and cost-conscious manner.
The tumor microenvironment state associates with response to HPV therapeutic vaccination in patients with respiratory papillomatosis. Norberg, SM, et al. Science Translational Medicine. 2023. https://www.science.org/doi/full/10.1126/scitranslmed.adj0740
Initial safety results and immune responses induced by a novel human papillomavirus (HPV)-specific gorilla adenovirus immunotherapy vaccine, PRGN-2009, in patients with advanced HPV-associated cancers. Floudas C, et al. Journal for ImmunoTherapy of Cancer. 2021. http://dx.doi.org/10.1136/jitc-2021-SITC2021.483.
Preclinical study of a novel therapeutic vaccine for recurrent respiratory papillomatosis (PRGN-2012). Lee, M.Y., Metenou, S., Brough, D.E. et al. npj Vaccines. 2021. https://doi.org/10.1038/s41541-021-00348-x.
Characterization of a recombinant gorilla-adenovirus HPV therapeutic vaccine (PRGN-2009). Pellom, Samuel T. et al. JCI Insight. 2021. https://doi.org/10.1172/jci.insight.141912.
A gorilla adenovirus-based vaccine against Zika virus induces durable immunity and confers protection in pregnancy. Hassan, et al. (2019) Cell Reports. 28:2634-2646.
Genetic vaccine for respiratory syncytial virus provides protection without disease potentiation. Johnson, et al. (2014) Mol Ther. 22:196-205.
Adenoviruses isolated from wild gorillas are closely related to human species C viruses. McVey, et al. (2013) Virology. 444:119-123.
Utilization of site-specific recombination for generating therapeutic protein producing cell lines. Campbell, et al. (2010) Molecular Biotechnology. 45:199-202. PMID: 20300883.
Phage Bxb1 integrase mediates highly efficient site-specific recombination in mammalian cells. Russell, et al. (2006) Biotechniques. 40:460, 462, 464. PMID: 16629393.
Phase 1/1b study of PRGN-3005 autologous UltraCAR-T cells manufactured overnight for infusion next day to advanced stage platinum resistant ovarian cancer patients. Liao, J.B., et al. (2023) American Society of Clinical Oncology (ASCO) Annual Meeting. Abstract 5590.
Next generation UltraCAR-T® cells with intrinsic chgeckpoint inhibition and overnight manufacturing overcome suppressive tumor microenvironment leading to sustained antitumor activity. Zenere, G., et al. (2023) American Association for Cancer Research (AACR) Annual Meeting. Abstract 1791.
Phase 1/1b Safety Study of PRGN-3006 UltraCAR-T in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndromes. Sallman, D., et al. (2022) 64th Annual Meeting and Exposition of the American Society of Hematology (ASH). Abstract 4633.
A Phase1/1b Dose Escalation/Dose Expansion Study of PRGN-3007 UltraCAR-T Cells in Patients with Advanced Hematologic and Solid Tumor Malignancies. Ibarz, J., et al. (2022) 64th Annual Meeting and Exposition of the American Society of Hematology (ASH). Abstract 3334.
Incorporation of intrinsic checkpoint blockade enhances functionality of multigenic autologous UltraCAR-T cells manufactured using non-viral gene delivery and rapid manufacturing process. Chan, T., et al. (2022) American Association for Cancer Research (AACR) Annual Meeting. Abstract 2821.
Phase 1/1b Safety Study of PRGN-3006 UltraCAR-T in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndromes. Sallman, D., et al. (2021) 63rd Annual Meeting and Exposition of the American Society of Hematology (ASH). Abstract 825.
Preclinical Evaluation of PRGN-3007, a Non-Viral, Multigenic, Autologous ROR1 UltraCAR-T Therapy with Novel Mechanism of Intrinsic PD-1 Blockade for Treatment of Hematological and Solid Cancers. Chan, T., et al. (2021) 63rd Annual Meeting and Exposition of the American Society of Hematology (ASH). Abstract 1694.
A Phase 1/1b Safety Study of PRGN-3006 UltraCAR-T in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndrome. Sallman, D. et al. Blood (2020) 136 (Supplement 1): 17. 62nd Annual Meeting and Exposition of the American Society of Hematology. Abstract 2864.
PRGN-3005 UltraCAR-T: Multigenic CAR-T cells generated using non-viral gene delivery and rapid manufacturing process for the treatment of ovarian cancer. Chan, T., et al. (2020) American Association for Cancer Research (AACR) Virtual Annual Meeting II. Abstract 6593.
Preclinical characterization of PRGN-3006 UltraCAR-T for the Treatment of AML and MDS: non-viral, multigenic autologous CAR-T cells administered one day after gene transfer. Chan, T., et al. (2019) Blood. 134:2660.
Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Hurton, et al. (2016) Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7788-E7797. Epub 2016 Nov 14. PMID: 27849617.
Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Hackett, et al. (2013) Translational Research. 161:265-83. PMID: 23313630.
Genome-wide Profiling Reveals Remarkable Parallels Between Insertion Site Selection Properties of the MLV Retrovirus and the piggyBac Transposon in Primary Human CD4(+) T Cells. Gogol-Döring, et al. (2016) Mol Ther. 2016 Mar;24(3):592-606. doi: 10.1038/mt.2016.11. Epub 2016 Jan 12. PMID: 26755332.
Comparison of lentiviral and sleeping beauty mediated αβ T cell receptor gene transfer. Field, et al. (2013) PLoS One. 2013 Jun 28;8(6):e68201. doi: 10.1371/journal.pone.0068201. Print 2013. PMID: 23840834.
Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2, and piggyBac transposons in human primary T cells. Huang, et. al (2010) Mol Ther. 2010 Oct;18(10):1803-13. doi: 10.1038/mt.2010.141. Epub 2010 Jul 6. PMID: 20606646.